Artificial water channels
Ratul Chowdhury, Tingwei Ren, Manish Shankla, Karl Decker, Matthew Grisewood, Jeevan Prabhakar, Carol Baker, John H. Golbeck, Aleksei Aksimentiev, Manish Kumar, and Costas D. Maranas Nature Communications (2018)
Monodispersed angstrom-size pores embedded in a suitable matrix are promising for highly selective membrane-based separations. They can provide substantial energy savings in water treatment and small molecule bioseparations. Such pores present as membrane proteins (chiefly aquaporin-based) are commonplace in biological membranes but difficult to implement in synthetic industrial membranes and have modest selectivity without tunable selectivity. Here we present PoreDesigner, a design workflow to redesign the robust beta-barrel Outer Membrane Protein F as a scaffold to access three specific pore designs that exclude solutes larger than sucrose (>360 Da), glucose (>180 Da), and salt (>58 Da) respectively. PoreDesigner also enables us to design any specified pore size (spanning 3–10 Å), engineer its pore profile, and chemistry. These redesigned pores may be ideal for conducting sub-nm aqueous separations with permeabilities exceeding those of classical biological water channels, aquaporins, by more than an order of magnitude at over 10 billion water molecules per channel per second.
Karl Decker, Martin Page, Ashley Boyd, Irene MacAllister, Mark Ginsberg, and Aleksei Aksimentiev ACS Biomaterials Science & Engineering (2017)
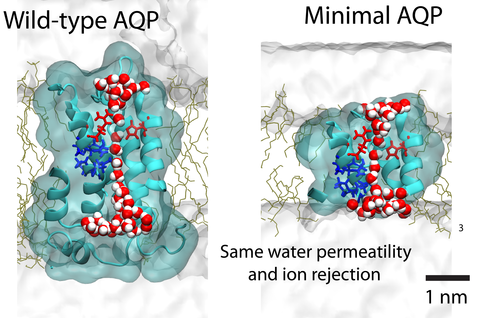
Aquaporin (AQP) proteins function as highly efficient water transport channels that support homeostasis in many types of living cells. Their structure-function relationships have been characterized extensively in fundamental and applied research, primarily via structural analysis, mutational studies, and computational approaches. The present study evaluates the effects of progressive truncations on the permeability and ionic conductivity of AQP-1 (bovine). The use of truncations to determine critical features has not been considered previously, as physical truncation of AQP is likely not technically feasible due to the ornate arrangement of six interwoven alpha helices in a single pore structure. However, structures not obtainable through protein assembly can be realized via synthetic chemistry approaches and studied using molecular dynamics (MD) simulations. Here, we apply the MD method to characterize the permeability of AQP variants truncated along the pore axis from both cytoplasmic and extracellular sides of the channel. The simulation results suggest that AQP-1 can retain its function even after deletion of up to 50% of the channel’s length, representing 50% of proteins’s molecular mass. Deletions such as these are expected to greatly simplify future biomimicry efforts of reproducing the AQP functionality using synthetic macromolecules. This study demonstrates the potential of in silico approaches to support the creation of streamlined functional analogs of biological molecular machines.
Yue-xiao Shen, Wen Si, Mustafa Erbakan, Karl Decker, Rita De Zorzi, Patrick O. Saboe, You Jung Kang, Sheereen Majd, Peter J. Butler, Thomas Walz, Aleksei Aksimentiev, Jun-li Hou, and Manish Kumar Proceedings of the National Academy of Science (2015)
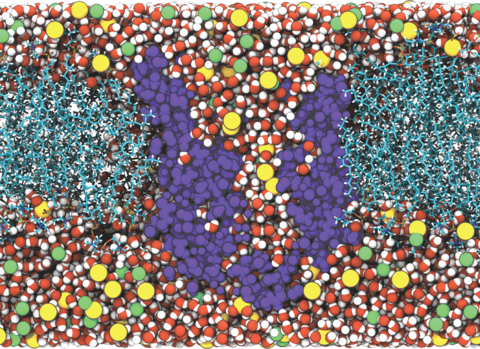
Bioinspired artificial water channels aim to combine the high permeability and selectivity of biological aquaporin (AQP) water channels with chemical stability. We characterized a new architecture of artificial water channels, peptide-appended pillar[5]arenes (PAPs). The average single-channel osmotic water permeability for PAPs is 1.0(± 0.3)×10−14 cm3/s or 3.5(± 1.0)×108 water molecules/s, which is in the range of AQPs (3.4~40.3×108 water molecules/s) and their current synthetic analogs, carbon nanotubes (CNTs, 9.0×108 water molecules/s). This permeability is an order of magnitude higher than first-generation artificial water channels (20 to ~107 water molecules/s). Furthermore, within lipid bilayers, PAP channels can self-assemble into two-dimensional arrays. Importantly for permeable membrane design, the pore density of PAP channel arrays (~2.6×105 pores/μm2) is two orders of magnitude higher than CNT membranes (0.1~2.5×103 pores/μm2). PAP channels thus combine the advantages of biological channels and CNTs and improve upon them through their relatively simple synthesis, chemical stability and propensity to form arrays.