Resolving Isomeric Posttranslational Modifications Using a Biological Nanopore as a Sensor of Molecular Shape
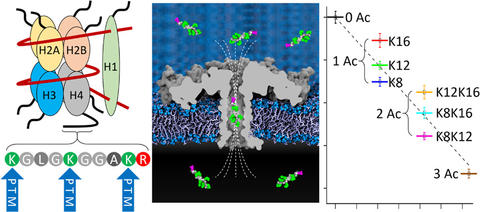
The chemical nature and precise position of posttranslational modifications (PTMs) in proteins or peptides are crucial for various severe diseases, such as cancer. State-of-the-art PTM diagnosis is based on elaborate and costly mass-spectrometry or immunoassay-based approaches, which are limited in selectivity and specificity. Here, we demonstrate the use of a protein nanopore to differentiate peptides─derived from human histone H4 protein─of identical mass according to the positions of acetylated and methylated lysine residues. Unlike sequencing by stepwise threading, our method detects PTMs and their positions by sensing the shape of a fully entrapped peptide, thus eliminating the need for controlled translocation. Molecular dynamics simulations show that the sensitivity to molecular shape derives from a highly nonuniform electric field along the pore. This molecular shape-sensing principle offers a path to versatile, label-free, and high-throughput characterizations of protein isoforms.