Long-Range Conductivity in Proteins Mediated by Aromatic Residues
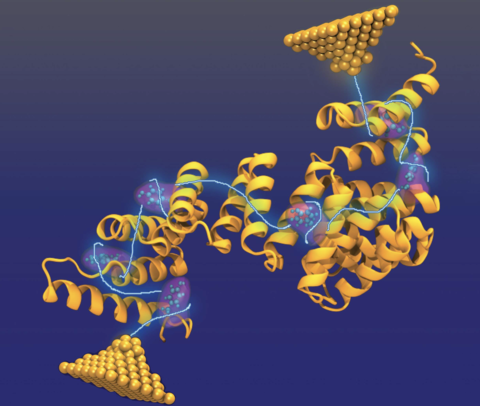
Single-molecule measurements show that many proteins, lacking any redox cofactors, nonetheless exhibit electrical conductance on the order of a nanosiemen over 10 nm distances, implying that electrons can transit an entire protein in less than a nanosecond when subject to a potential difference of less than 1 V. This is puzzling because, for fast transport (i.e., a free energy barrier of zero), the hopping rate is determined by the reorganization energy of approximately 0.8 eV, and this sets the time scale of a single hop to at least 1 μs. Furthermore, the Fermi energies of typical metal electrodes are far removed from the energies required for sequential oxidation and reduction of the aromatic residues of the protein, which should further reduce the hopping current. Here, we combine all-atom molecular dynamics (MD) simulations of non-redox-active proteins (consensus tetratricopeptide repeats) with an electron transfer theory to demonstrate a molecular mechanism that can account for the unexpectedly fast electron transport. According to our MD simulations, the reorganization energy produced by the energy shift on charging (the Stokes shift) is close to the conventional value of 0.8 eV. However, the non-ergodic sampling of molecular configurations by the protein results in reaction-reorganization energies, extracted directly from the distribution of the electrostatic energy fluctuations, that are only ∼0.2 eV, which is small enough to enable long-range conductivity, without invoking quantum coherent transport. Using the MD values of the reorganization energies, we calculate a current decay with distance that is in agreement with experiment.