PoreDesigner for tuning solute selectivity in a robust and highly permeable outer membrane pore
Monodispersed angstrom-size pores embedded in a suitable matrix are promising for highly selective membrane-based separations. They can provide substantial energy savings in water treatment and small molecule bioseparations. Such pores present as membrane proteins (chiefly aquaporin-based) are commonplace in biological membranes but difficult to implement in synthetic industrial membranes and have modest selectivity without tunable selectivity. Here we present PoreDesigner, a design workflow to redesign the robust beta-barrel Outer Membrane Protein F as a scaffold to access three specific pore designs that exclude solutes larger than sucrose (>360 Da), glucose (>180 Da), and salt (>58 Da) respectively. PoreDesigner also enables us to design any specified pore size (spanning 3–10 Å), engineer its pore profile, and chemistry. These redesigned pores may be ideal for conducting sub-nm aqueous separations with permeabilities exceeding those of classical biological water channels, aquaporins, by more than an order of magnitude at over 10 billion water molecules per channel per second.
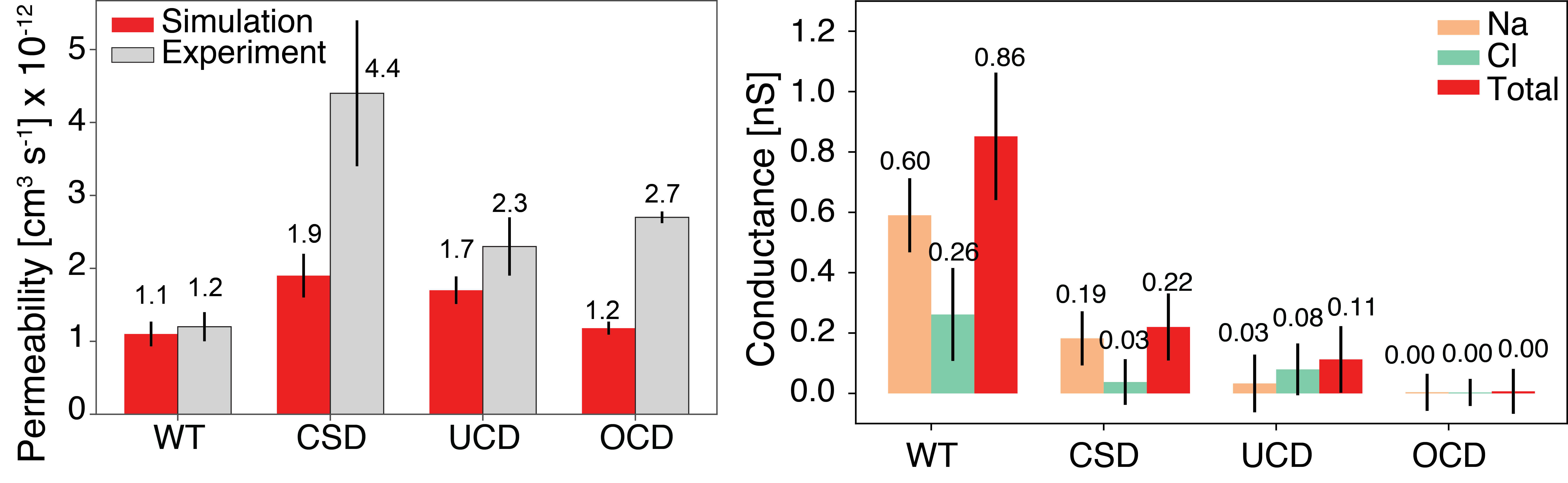
The osmotic permeability (left) and ionic conductance (right) of the wildtype Ompf trimer compared to the cork-screw (CSD), uniform pore closure (UCD), and off-center pore closure (OCD) designs mutants. The osmotic permeability was calculated in equilibrium conditions while the ionic conductance was measured with an applied 500 mV transmembrane bias.
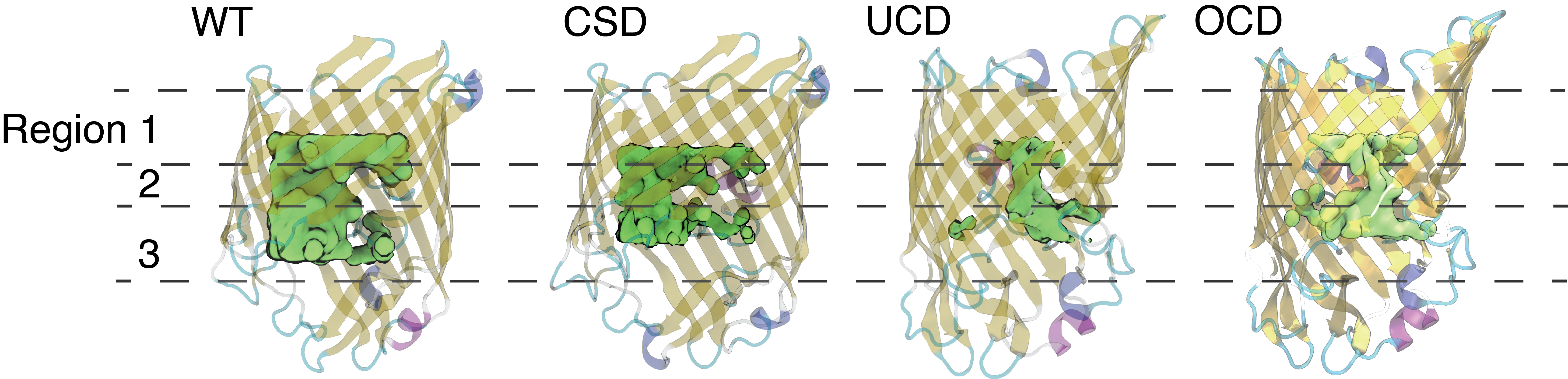
Average water occupancy inside the wildtype and mutant Ompf monomers illustrated as a green isosurface (0.3 g/cm3) inside the transparent protein channels.
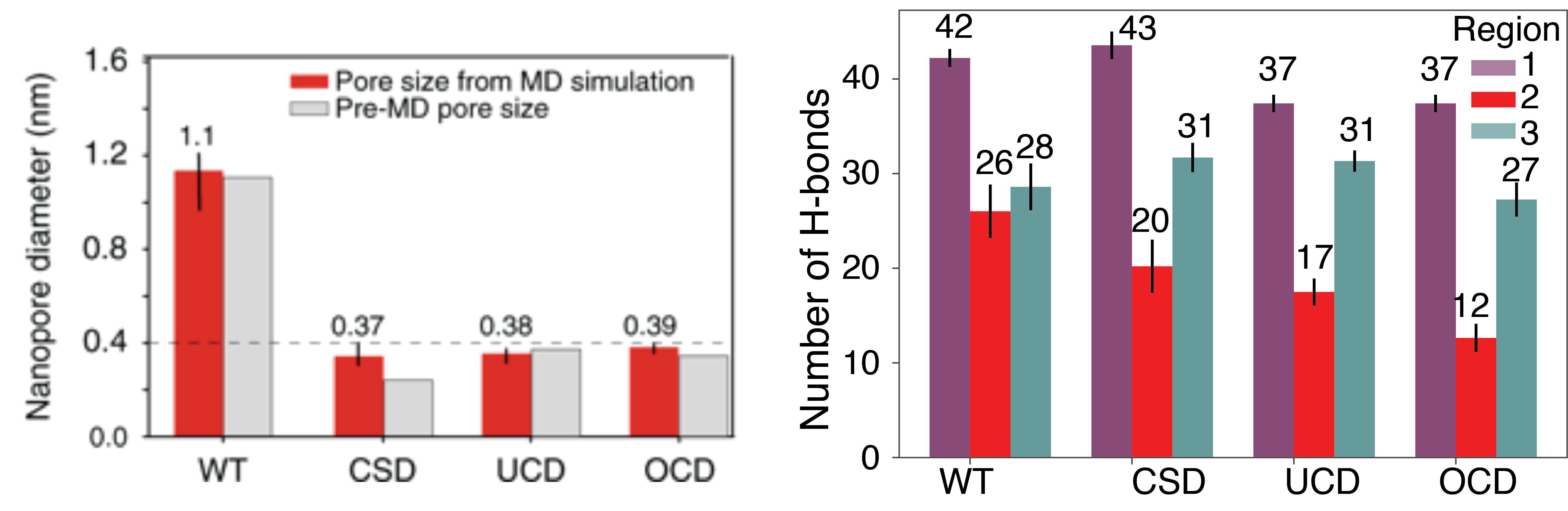
(Left) Wild type and mutant pore sizes before and after equilibration in MD simulations. The CSD Ompf channel is the only channel to deviate from the starting pore size. (Right) The average number of hydrogen bonds between water and the inside of the Ompf channel in each region of the pore shown in the image above.