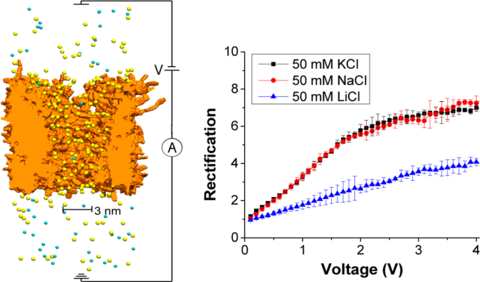
Rectifying nanopores feature ion currents that are higher for voltages of one polarity compared to the currents recorded for corresponding voltages of the opposite polarity. Rectification of nanopores has been found to depend on the pore opening diameter and distribution of surface charges on the pore walls as well as pore geometry. Very little is known, however, on the dependence of ionic rectification on the type of transported ions of the same charge. We performed experiments with single conically shaped nanopores in a polymer film and recorded current−voltage curves in three electrolytes: LiCl, NaCl, and KCl. Rectification degrees of the pores, quantified as the ratio of currents recorded for voltages of opposite polarities, were the highest for KCl and the lowest for LiCl. The experimental observations could not be explained by a continuum modeling based on the Poisson−Nernst−Planck equations. All-atom molecular dynamics simulations revealed differential binding between Li+, Na+, and K+ ions and carboxyl groups on the pore walls, resulting in changes to both the effective surface charge of the nanopore and cation mobility within the pore.

